![]()
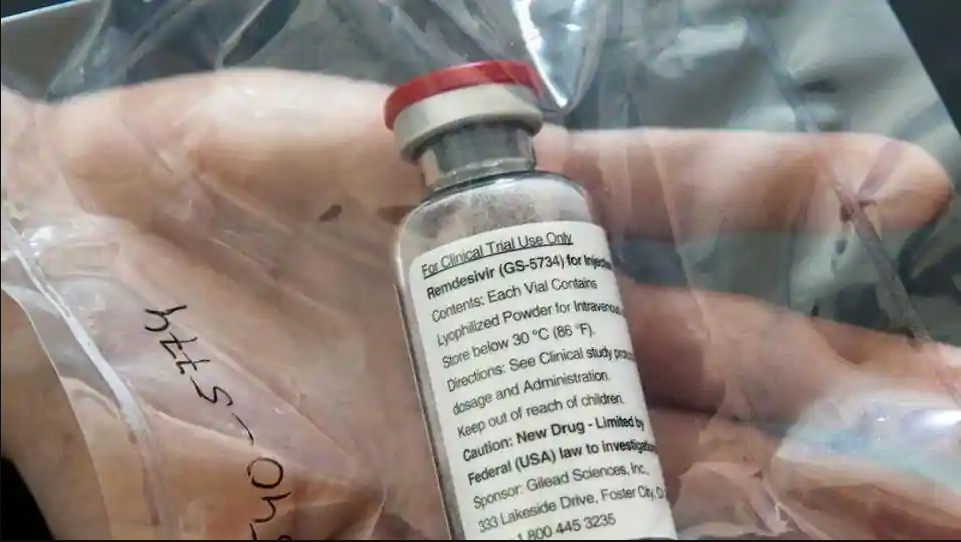
An ampule of Ebola drug Remdesivir is pictured during a news conference at the University Hospital Eppendorf (UKE) in Hamburg, Germany. (Reuters) COURTESY BY: https://www.hindustantimes.com/ Japan will fast-track a review of Gilead Sciences Inc’s antiviral drug remdesivir so that it can hopefully be approved for domestic COVID-19 patients a week after the U.S. firm’s filing for such approval, the health minister said on Saturday. Health Minister Katsunobu Kato’s comment comes after remdesivir was granted emergency use authorization by the U.S. Food and Drug Administration for COVID-19 on Friday. “I’ve heard…